Research groups
Our interdisciplinary lab is composed of 3 research groups that develop state-of-the-art projects within their specific field and share their complementary expertise within joint projects.
Biophotonics
(Amaury Badon)
Biophysics and Biomicrofluidics
(Pierre Nassoy)
Bioengineering and BioImaging
(Gaëlle Recher)
The development of 3D imaging/optical techniques and subsequent computational approaches allows novel ways of monitoring the growth of 3D cell cultures at large scale, with high resolution and in a harmless label-free manner.
The use of soft matter physics concepts and experimental approaches to investigate self-organization and dynamics of growing engineered tissues allows novels ways to decipher emergent properties and mechanosensitive responses of cellular assemblies.
The design, engineering and manufacture of microfabricated devices allows novel ways of manipulating cells, building self-assembled tissues, applying external biomechanical cues and imaging them for quantitative investigations.
Joint projects
Our interdisciplinary lab is composed of 3 research groups (link) that develop state-of-the-art projects within their specific field and share their complementary expertise within joint projects.
Incubascope
(Gaëlle Recher & Amaury Badon)
A low-cost, wide field microscope in an incubator for medium throughput monitoring of multicellular cultures.
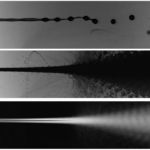
Light absorption based flow analyzer of cellular capsules
(Amaury Badon & Pierre Nassoy)
Collaborative projects

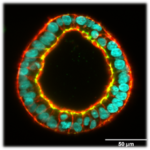
3D cell-based hollow liver capsules for drug screening assays
(P. Nassoy i collaboration with F. Saltel, BRIC, Bordeaux and M. Daujat/S. Gerbal, IRMB, Monpellier)
Mechanics of induced pluripotent stem cell cysts
(P. Nassoy with TreeFrog Therapeutics)
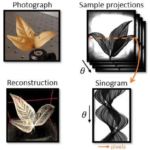
3D shaping of terahertz waves
(A. Badon with P. Mounaix (IMS, Bordeaux) and S. Gigan (LKB, Paris) )
To read more



