Principal investigator, DR1 – CNRS

Research interests/keywords
Multicellular spheroids and organoids represent the most versatile and promising in vitro systems to recapitulate model tumors and tissues. Ten years ago, we developed a microfluidic technique, namely the Cellular Capsule Technology, that allows to produce cellular aggregates encapsulated in hydrogel shells. Our current interests consist in i) developing an integrated platform for optimized production, sorting and analysis of spheroids/organoids, ii) studying the material properties of these growing tissues/tumors (mechanotransduction, self-organization, dynamical instability) from a physics perspective, iii) addressing fundamental biology questions and investigating medical applications (cell therapy) by using our ability to produce spheroids/organoids in a controlled and high throughput format; iv) contributing to the generation of digital twins of tissues by providing controlled engineered organoids and by devising assembloids for calibration and validation of mathematical models.
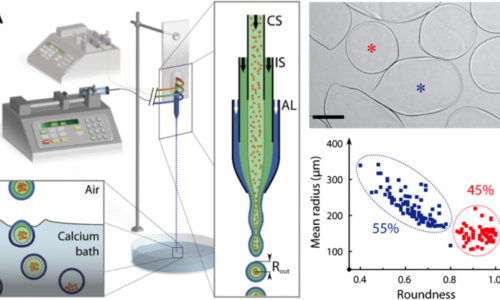
Production, sorting and analysis of cellular capsules
Our current efforts focus on optimizing the production of capsules (sphericity, monodispersity) and increasing the versatility of the CCT (shape, stiffness, chemical composition). We aim at designing integrated microfluidics-based lab-on-chips dedicated to the sorting and in situ manipulation, treatments and analysis of 3D cell cultures.
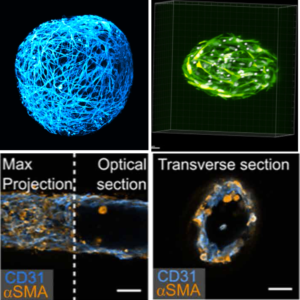
Biological and biomedical applications of cellular capsules
We use cellular capsules to generate iPSC-derived neuronal organoids, artificial liquid tumors comprising B lymphocytes and primary stromal cells, engineered liver modules or in vitro blood vessels, coined vesseloids. This allows us (together with our collaborators) to venture into cell therapy, chemotherapy assays, tumor vascularization, NASH modelling, vessel transplantation.
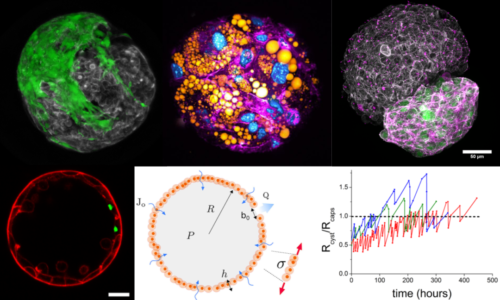
Biophysics of cellular capsules
The alginate capsules can serve as microcompartments to allow two (or more) types of cells to self-organize in a restricted space. Due to the elastic properties of the alginate gel, the capsules can also be viewed as a mechanical tool to apply an external pressure on the growing cell aggregates once they have reached 3D confluence. These compressive cues may lead to a phenotypic switch towards an invasive phenotype, to an enwrapping/spreading transition, to a mechanically-induced differentiation of progenitor cells, or to unusual pulsatile growth dynamics. We always aim for quantitative imaging and theoretical modelling relying on simple concepts inspired from soft matter physics to explain the observed behaviors.
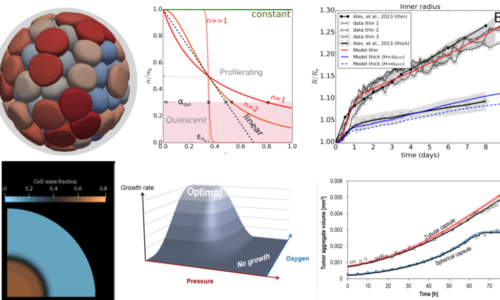
Capsules as a model system towards digital twins of tissues
Mathematical models of biological tissues prove increasingly useful in experimental data interpretation when analytical physical models are insufficient. However, these models often rely on a vast number of unknown parameters. In order to increase their predictive value, we pursue a bottom-up approach by usual the cellular capsules as simplified and controlled model tissues. By tuning the cell seeding density, the shape of the capsules, their stiffness, we may help calibrate the mathematical models and bring a reliable contribution to digital twins. This work is based on collaborations with the team of G. Sciumé (for equation-based models) and the one of D. Drasdo (for agent-based approaches).
Supervision
Postdoctoral scientists :
- Naveen Mekhileri
PhD students :
- Léon Rembotte (40 %)
- Anirban Jana (30 %)
- Lucas Suire (50 %)
- Fernanda Lopez Garcia (50%)
Engineers :
- Loïc Hermant
Selected publications
- Cohen PJR, Luquet E, Pletenka J, Leonard A, Warter E, Gurchenkov B, Carrere J, Rieu C, Hardouin J, Moncaubeig F, Lanero M, Quelennec E, Wurtz H, Jamet E, Demarco M, Banal C, Van Liedekerke P, Nassoy P, Feyeux M, Lefort N, Alessandri K. Engineering 3D micro-compartments for highly efficient and scale-independent expansion of human pluripotent stem cells in bioreactors. Biomaterials 295:122033.
- Alessandri K., Feyeux M., Gurchenkov B., Delgado C., Trushko A., Krause K-H., Vignjevic D., Nassoy P., Roux A. A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human Neuronal Stem Cells (hNSC). Lab Chip 13:1593-1604.
- Alessandri K., Sarangi B.R. , Gurchenkov V.V., Sinha B., Kiessling T.R., Fetler L., Rico F., Scheuring S., Lamaze C., Simon A., Geraldo S., Vignjevic D., Domejean H., Rolland L., Funfak A., Bibette J., Bremond N., Nassoy P. Cellular capsules as a tool for multicellular spheroid production and for investigating the mechanics of tumor progression in vitro. Natl. Acad. Sci. USA, 110 (37): 14843−14848.
- Sinha B., Köster D., Ruez R., Gonnord P., Bastiani M., Abankwa D., Stan R. V., Butler-Browne G., Vedie B., Johannes L., Morone N., Parton R. G., Raposo G., Sens P., Lamaze C., Nassoy P. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell, 144: 402-413.
- Merkel R., Nassoy P., Leung A., Ritchie K. and Evans E. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature 397: 50-53.


